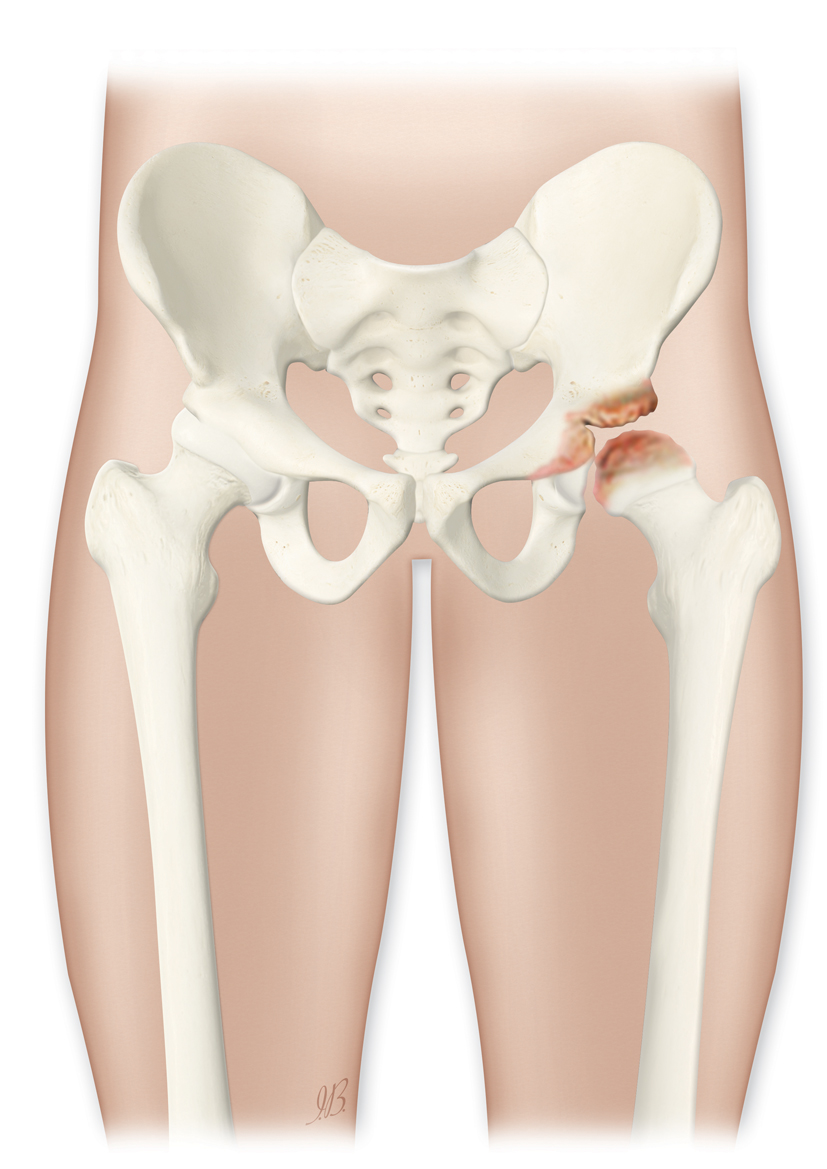
29. Mills S, Burroughs KE. 2020. Legg Calve Perthes Disease (Calves Disease). StatPearls Publishing, Treasure Island (FL). www.ncbi.nlm.nih.gov
30. Mayo Clinic. Legg-Calve-Perthes disease. www.mayoclinic.org
31. Brech CG, D’Andrea Greve JM et al. 2015. Conservative Treatment for Patients with Legg-Calve-Perthes Disease: Seven Years of Follow-up. J Nov Physiother; 5:1.
32. Arkander A, Sankar WN et al. 2009. Conservative versus surgical treatment of late-onset Legg-Calve-Perthes Disease: a radiographic comparison at skeletal maturity. J Child Orthop; 3(1):21-5.
33. Boston Children’s Hospital. Legg-Calve-Perthes Disease. www.childrenshospital.org
34. Southampton Children’s Hospital. Perthes disease. www.uhs.nhs.uk
35. Singh A, Srivastava RN et al. 2014. Management of Late Onset Perthes: Evaluation of Distraction by External Fixator – 5-Year Follow-Up. Adv Orthop;135236
36. Malheiros Luzo CA, Guarniero et al. 2016. Initial experience of use of an articulated external fixator in treating Legg-Calvé-Perthes disease by means of orthodiastasis during the active phase of the disease. Rev Bras Ortop; 51(3):337-345
 Share on facebook
Share on facebook
 Share on twitter
Share on twitter
 Share on linkedin
Share on linkedin
 Share on email
Share on email